CAR-T Cells, Nuclear Fusion, CRISPR, Conscious AI, 2024 DeepTech - SpecialeFocus #13
Seasons Greetings from Team Speciale ;)
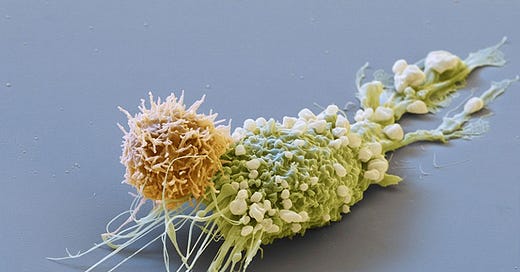
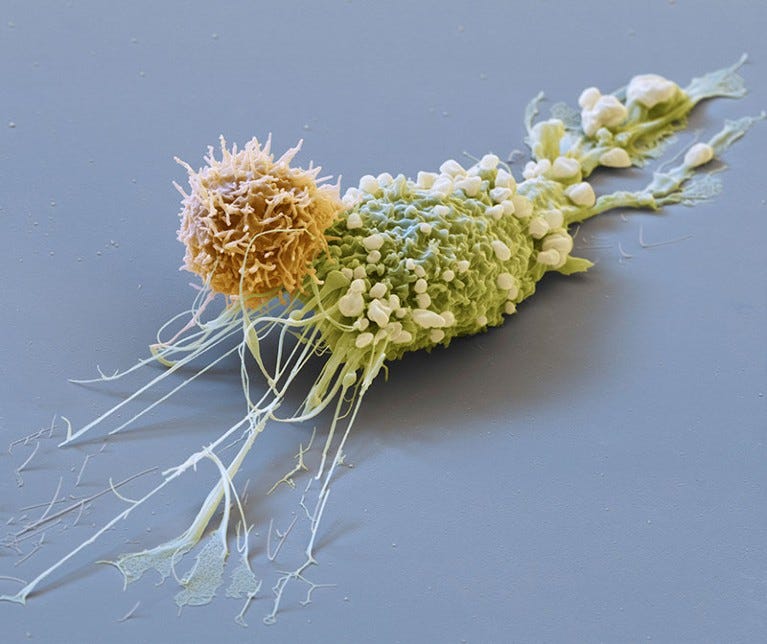
Producing CAR-T cells within the patient’s body - Next Frontier in Cancer Therapeutics
Scientists are making progress towards producing chimeric antigen receptor T cells (CAR-T cells) within the human body, potentially making cancer therapies more accessible. CAR-T treatment involves engineering T cells outside the body to target cancer cells and then reintroducing them, but the process is expensive and complex.
Recent results presented at the American Society of Hematology suggest that a virus injection could be used to genetically engineer T cells inside the body, eliminating the need for cell extraction and purification. Two companies, Interius BioTherapeutics and Umoja Biopharma, reported successful experiments on monkeys targeting B cells, with plans to request FDA trials on humans. The technique could potentially reduce the cost of CAR-T therapies, currently exceeding half a million dollars.
However, challenges remain, including ensuring the precision targeting of T cells and addressing regulatory concerns. Other approaches, such as using donor cells, are also being explored. Sana Biotechnology, which had been developing a virus to generate CAR-T cells in the body, recently scaled back that program to focus on other projects.
Read more here.
Sustained Nuclear Fusion, Yet Againnnn!
On Dec. 5, a team at LLNL’s National Ignition Facility (NIF) conducted the first controlled fusion experiment in history to demonstrate a sustained fusion reaction producing more energy from fusion than the laser energy used to drive it.
Physicist Annie Kritcher played a key role in the US Department of Energy's National Ignition Facility (NIF) achieving a significant breakthrough: compressing atoms tightly enough to induce nuclear fusion, generating more energy than consumed—a goal that had eluded laboratories worldwide for decades. The NIF's success in achieving ignition came after a decade-long delay. Kritcher, as the lead designer, and her team aimed to prove that the NIF could reliably achieve ignition. In July, the facility's 192 laser beams delivered 2.05 megajoules of energy, resulting in a fusion reaction that produced a record 3.88 megajoules of fusion energy. This marked the first time a lab had produced a fusion reaction with more energy output than input.
Building on their success in July, Annie Kritcher and her team conducted two additional ignition experiments in October, achieving success in four out of the last six attempts. With plans for even greater yields in the coming year, scientists at the facility have opened up new avenues for research, contributing to a surge of optimism regarding the prospects of fusion energy. Read more.
FDA approves the First CRISPR based therapy!
The U.S. Food and Drug Administration (FDA) has approved two groundbreaking cell-based gene therapies, Casgevy and Lyfgenia, for the treatment of sickle cell disease (SCD) in patients aged 12 and older. Casgevy is the first FDA-approved therapy utilizing CRISPR/Cas9, a genome editing technology, to modify patients' hematopoietic stem cells. The edited cells are then transplanted back into the patient, increasing the production of fetal hemoglobin and preventing the sickling of red blood cells. Lyfgenia, a cell-based gene therapy using a lentiviral vector, modifies patients' blood stem cells to produce HbAT87Q, a gene-therapy-derived hemoglobin that reduces the risk of sickling in red blood cells.
Both therapies are made from the patients' own blood stem cells and are administered as a one-time, single-dose infusion as part of a hematopoietic stem cell transplant. Patients undergo myeloablative conditioning (high-dose chemotherapy) before receiving the modified cells. The FDA granted Priority Review, Orphan Drug, Fast Track, and Regenerative Medicine Advanced Therapy designations to both Casgevy and Lyfgenia. The approval represents a significant advance in using innovative cell-based gene therapies to address severe health conditions, with a focus on improving public health outcomes. Patients receiving Lyfgenia are subject to lifelong monitoring due to the risk of hematologic malignancies.
Casgevy was developed by Vertex Pharmaceuticals Inc., and Lyfgenia was developed by Bluebird Bio Inc. Both therapies aim to address the underlying genetic cause of sickle cell disease, providing a potential life-changing treatment for patients with this debilitating and life-threatening blood disorder. Read more.
The Great AI Consciousness Conundrum
The invitation to speak at the Conference on Neural Information Processing Systems (NeurIPS) came as a surprise to David Chalmers, a leading authority on consciousness. The conference organizers sought his insights on artificial intelligence (AI) consciousness following claims that Google's AI system, LaMDA, had achieved consciousness. Chalmers, known for his work on the philosophy of mind, discussed the challenges of defining and identifying consciousness, emphasizing the difficulty of making objective assessments due to its inherently subjective nature.
Chalmers estimated that there was a greater than one-in-five chance of developing conscious AI in the next decade, prompting serious discussions among AI experts about the implications of machine consciousness. The moral and ethical considerations surrounding AI consciousness involve the risk of unintentionally mistreating or causing harm to a conscious being and the potential for overlooking unconscious AI, compromising human safety.
Researchers like Liad Mudrik, a neuroscientist at Tel Aviv University, have made progress in understanding human consciousness through experiments that manipulate conscious experiences. While there have been advancements in detecting consciousness in humans using brain stimulation, applying such tests to AI presents challenges due to the absence of a physical brain.
Various theories about consciousness propose different criteria, with some focusing on the brain's software architecture, while others emphasize the role of physical hardware. The lack of a unified theory complicates efforts to test for AI consciousness. Some propose language-based tests, like Susan Schneider's "artificial consciousness test," while others advocate for understanding the internal workings of AI systems.
Collaborative efforts among consciousness researchers have led to adversarial collaborations, where different theories are tested against each other through neuroscience experiments. Mudrik's team, for example, has worked on a consciousness "report card" that combines markers from various theories to assess AI consciousness. The absence of these markers in current large language models like LaMDA suggests they lack key elements associated with consciousness.
As AI continues to advance, the question of AI consciousness becomes increasingly relevant, and researchers grapple with the practical and ethical implications of potentially conscious machines.
Read more of this fascinating discourse here.
Seasons Greetings from Team Speciale ;)
That’s it for 2023 folks! Before we break for the year, we wanted to leave with what’s exciting in DeepTech for 2024. Check this out.
We’ll see you soon with another bunch of WOW reads specially curated for the curious you.