Private Moon Landing, SORA, Neuralink, SLIM, CAR-T Cells - SpecialeFocus #15
To the Moon and beyond...
The World’s First Private Moon Landing - Not Perfect, but Just Enough
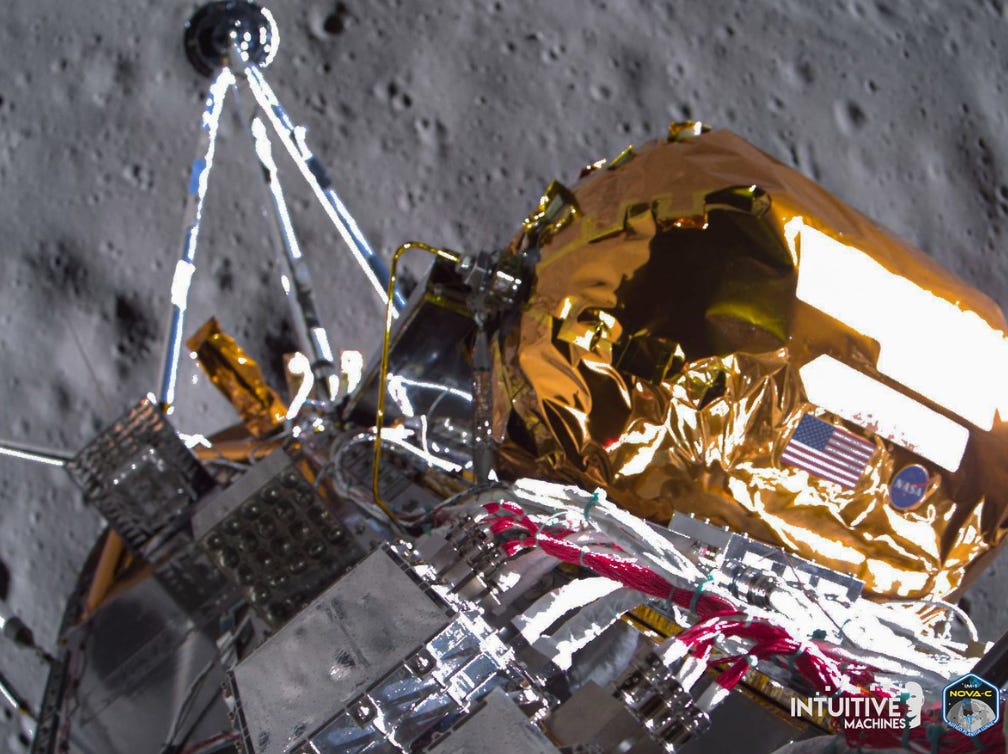
Intuitive Machines made history with the successful landing of its spacecraft, Odysseus, near the lunar south pole, marking the first privately built and operated craft to achieve such a feat on the moon. Despite encountering a setback where the craft landed on its side, thereby altering the positioning of its solar panels, Odysseus perseveres in transmitting valuable science data and imagery. Although this resulted in some uncertainty regarding the remaining battery life, estimated at 10-20 hours out of the expected 7-10 days of operation, the mission's objectives remain achievable.
Despite challenges, Odysseus continues to contribute to our understanding of the lunar environment, demonstrating resilience and the ingenuity of its creators. Additionally, the success of the landing owes much to the swift and resourceful response of the flight controllers, who adeptly improvised a navigation solution when the onboard laser range finders encountered issues due to human error.
Overall, this accomplishment stands as a testament to human innovation and collaboration, marking a significant milestone in space exploration for the benefit of all humanity.
OpenAI’s SORA Changed Video Creation Forever
Sora is a tool that transforms fuzzy noise into videos. It can create complete videos or extend existing ones seamlessly. Even if something temporarily disappears from view, Sora maintains continuity. Similar to GPT models, Sora employs a specialized design for efficient performance. It breaks down videos and images into smaller units called patches, facilitating handling of various visual content. Sora draws from research on DALL·E and GPT, enabling it to accurately follow user instructions for video creation. It can convert text into videos, animate still images, and enhance existing videos by adding frames. Sora represents a step forward in enabling computers to understand and replicate real-world scenarios, advancing the journey towards highly intelligent AI.
Neuralink Implants Brain Chip in FIRST Human
Elon Musk's Neuralink has successfully implanted its first brain-chip device into a human patient, with Musk reporting positive progress in the patient's recovery. The implant is designed to detect neuron spikes, marking a significant step in Neuralink's mission to aid individuals with paralysis and neurological disorders. The U.S. FDA granted clearance for Neuralink's human trial, allowing the startup to proceed with its innovative approach. The implant, known as Telepathy, aims to enable users to control computer functions using their thoughts alone. Neuralink's technology utilizes ultra-fine threads to transmit brain signals, with the PRIME Study assessing the safety of its wireless brain-computer interface and surgical procedures.
SLIM Survives the Lunar Night!
Japan's SLIM spacecraft, despite not being designed for it, has survived the harsh lunar night and resumed communication with Earth. Its systems, including communications, onboard computer, and solar panels, are believed to be functioning. Despite challenges, including an upside-down landing and reduced sunlight, SLIM's electronics remain operational. Recent mission imagery reveals new lunar terrain, indicating ongoing scientific activity. SLIM has successfully completed its main and extended mission objectives, showcasing resilience in the harsh lunar environment. Read more here.
First Patient Declared Cancer Free Using India’s CAR-T Therapy
India's drug regulator, CDSCO, approved CAR-T cell therapy for commercial use a few months ago, offering hope to cancer patients. The therapy, which reprograms the immune system to fight cancer, has been successful for Dr. V K Gupta, a gastroenterologist in Delhi. Gupta accessed the therapy at a significantly lower cost in India compared to abroad. Doctors at Tata Memorial Hospital, where Gupta underwent the procedure, declared him free of cancer cells, marking a significant milestone. While the therapy shows promise, its success rate is still being assessed, with initial findings suggesting better survival chances for early-stage cancer patients. Developed by ImmunoACT, the therapy focuses on treating B-cell cancers and has been approved for commercial use in India since October 2023.
That’s it folks. We’ll leave you with this for the month and come back soon with another set of wow reads specially curated for the curious you!
Until then, Team Speciale signing off!